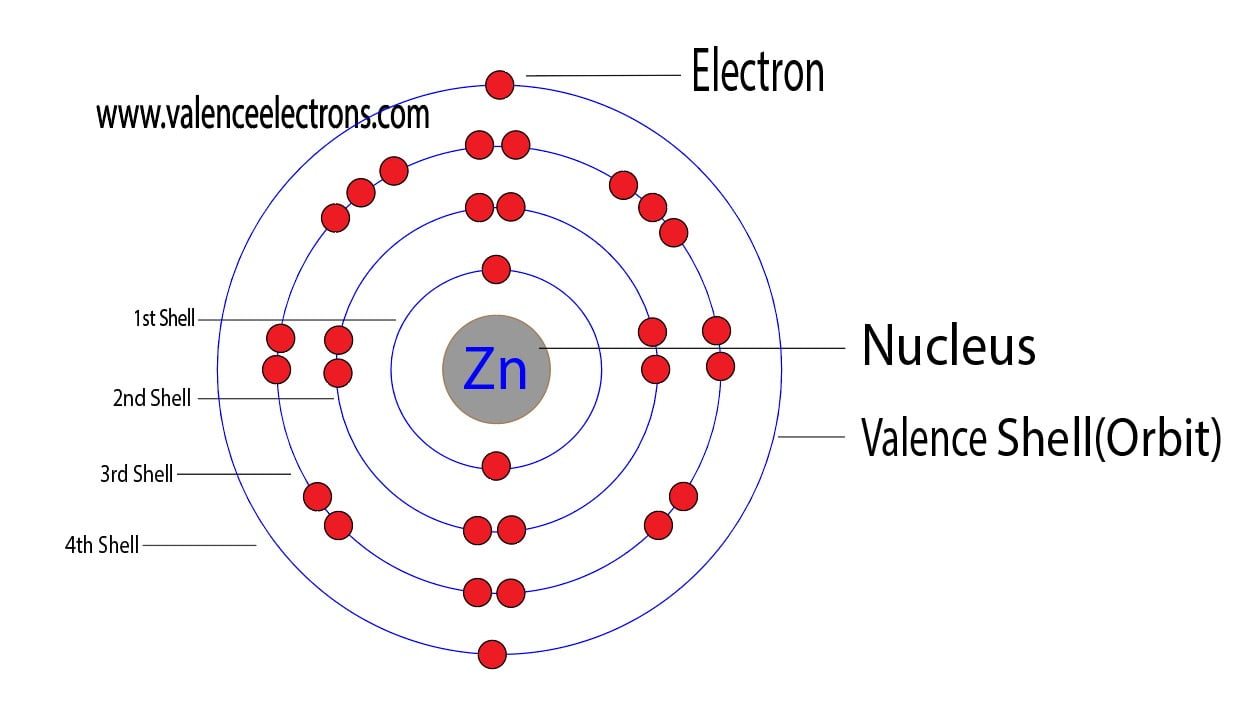
How Many Electrons Does Zinc Lose . Zinc is the metal transition element and hence it belongs to the d. Zinc atom electron configuration through orbit. zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. valency of zinc. table of contents. The precise and accurate valency of zinc is +2. therefore, the number of electrons in neutral atom of zinc is 30. Each electron is influenced by the electric fields produced by the positive nuclear charge. another implication is that zinc tends to lose its valence electrons to achieve a stable electron configuration. This means that neutral zinc atom has a total of. By losing its two 4s electrons, zinc. What is the electron configuration of zinc? in the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them.
from valenceelectrons.com
Zinc atom electron configuration through orbit. valency of zinc. By losing its two 4s electrons, zinc. The precise and accurate valency of zinc is +2. therefore, the number of electrons in neutral atom of zinc is 30. in the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Each electron is influenced by the electric fields produced by the positive nuclear charge. zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. Zinc is the metal transition element and hence it belongs to the d. another implication is that zinc tends to lose its valence electrons to achieve a stable electron configuration.
How Many Valence Electrons Does Zinc (Zn) Have?
How Many Electrons Does Zinc Lose Zinc is the metal transition element and hence it belongs to the d. another implication is that zinc tends to lose its valence electrons to achieve a stable electron configuration. in the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Zinc is the metal transition element and hence it belongs to the d. By losing its two 4s electrons, zinc. Each electron is influenced by the electric fields produced by the positive nuclear charge. The precise and accurate valency of zinc is +2. zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. What is the electron configuration of zinc? This means that neutral zinc atom has a total of. table of contents. valency of zinc. therefore, the number of electrons in neutral atom of zinc is 30. Zinc atom electron configuration through orbit.
From valenceelectrons.com
How many protons, neutrons and electrons does zinc have? How Many Electrons Does Zinc Lose Zinc atom electron configuration through orbit. By losing its two 4s electrons, zinc. Zinc is the metal transition element and hence it belongs to the d. another implication is that zinc tends to lose its valence electrons to achieve a stable electron configuration. What is the electron configuration of zinc? zinc is located in period 4, group 12. How Many Electrons Does Zinc Lose.
From elchoroukhost.net
Zinc Periodic Table Protons Neutrons Electrons Elcho Table How Many Electrons Does Zinc Lose Zinc atom electron configuration through orbit. in the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. therefore, the number of electrons in neutral atom of zinc is 30. table of contents. What is the electron configuration of zinc? This means that neutral zinc atom. How Many Electrons Does Zinc Lose.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Zinc Have? How Many Electrons Does Zinc Lose table of contents. valency of zinc. zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. What is the electron configuration of zinc? Zinc atom electron configuration through orbit. By losing its two 4s electrons, zinc. another implication is that zinc tends to lose its valence. How Many Electrons Does Zinc Lose.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? How Many Electrons Does Zinc Lose By losing its two 4s electrons, zinc. table of contents. another implication is that zinc tends to lose its valence electrons to achieve a stable electron configuration. therefore, the number of electrons in neutral atom of zinc is 30. zinc is located in period 4, group 12 of the periodic table and has an atomic number. How Many Electrons Does Zinc Lose.
From www.youtube.com
How many valence electrons are in zinc? YouTube How Many Electrons Does Zinc Lose What is the electron configuration of zinc? By losing its two 4s electrons, zinc. Each electron is influenced by the electric fields produced by the positive nuclear charge. in the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. table of contents. Zinc atom electron configuration. How Many Electrons Does Zinc Lose.
From www.slideshare.net
10 28 How Many Electrons Do Atoms Gain Lose How Many Electrons Does Zinc Lose Each electron is influenced by the electric fields produced by the positive nuclear charge. What is the electron configuration of zinc? therefore, the number of electrons in neutral atom of zinc is 30. another implication is that zinc tends to lose its valence electrons to achieve a stable electron configuration. table of contents. in the case. How Many Electrons Does Zinc Lose.
From chemistrytalk.org
The Zesty Element Zinc ChemTalk How Many Electrons Does Zinc Lose What is the electron configuration of zinc? in the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. table of contents. The precise and accurate valency of zinc is +2. therefore, the number of electrons in neutral atom of zinc is 30. Zinc atom electron. How Many Electrons Does Zinc Lose.
From thefitnessmanual.com
How Many Electrons Does Zinc Have TheFitnessManual How Many Electrons Does Zinc Lose Each electron is influenced by the electric fields produced by the positive nuclear charge. valency of zinc. By losing its two 4s electrons, zinc. in the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. another implication is that zinc tends to lose its valence. How Many Electrons Does Zinc Lose.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Zinc (Zn How Many Electrons Does Zinc Lose in the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. valency of zinc. The precise and accurate valency of zinc is +2. table of contents. zinc is located in period 4, group 12 of the periodic table and has an atomic number equal. How Many Electrons Does Zinc Lose.
From www.slideshare.net
10 28 How Many Electrons Do Atoms Gain Lose How Many Electrons Does Zinc Lose This means that neutral zinc atom has a total of. another implication is that zinc tends to lose its valence electrons to achieve a stable electron configuration. table of contents. The precise and accurate valency of zinc is +2. By losing its two 4s electrons, zinc. therefore, the number of electrons in neutral atom of zinc is. How Many Electrons Does Zinc Lose.
From valenceelectrons.com
How many protons, neutrons and electrons does zinc have? How Many Electrons Does Zinc Lose therefore, the number of electrons in neutral atom of zinc is 30. The precise and accurate valency of zinc is +2. Zinc is the metal transition element and hence it belongs to the d. zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. Zinc atom electron configuration. How Many Electrons Does Zinc Lose.
From zrlcourseworkqgi.web.fc2.com
How to write ions How Many Electrons Does Zinc Lose What is the electron configuration of zinc? table of contents. in the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. valency of zinc. another implication is that zinc tends to lose its valence electrons to achieve a stable electron configuration. The precise and. How Many Electrons Does Zinc Lose.
From www.slideserve.com
PPT PS4 The Atom & the Periodic Table PowerPoint Presentation ID How Many Electrons Does Zinc Lose What is the electron configuration of zinc? Zinc is the metal transition element and hence it belongs to the d. The precise and accurate valency of zinc is +2. table of contents. Each electron is influenced by the electric fields produced by the positive nuclear charge. This means that neutral zinc atom has a total of. another implication. How Many Electrons Does Zinc Lose.
From www.chegg.com
Solved Looking at this figure, how many electrons do How Many Electrons Does Zinc Lose in the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. What is the electron configuration of zinc? Each electron is influenced by the electric fields produced by the positive nuclear charge. Zinc is the metal transition element and hence it belongs to the d. another. How Many Electrons Does Zinc Lose.
From valenceelectrons.com
How many protons, neutrons and electrons does zinc have? How Many Electrons Does Zinc Lose Each electron is influenced by the electric fields produced by the positive nuclear charge. This means that neutral zinc atom has a total of. Zinc atom electron configuration through orbit. What is the electron configuration of zinc? another implication is that zinc tends to lose its valence electrons to achieve a stable electron configuration. table of contents. Zinc. How Many Electrons Does Zinc Lose.
From fity.club
Zinc Protons Neutrons Electrons Electron Configuration How Many Electrons Does Zinc Lose zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. What is the electron configuration of zinc? therefore, the number of electrons in neutral atom of zinc is 30. another implication is that zinc tends to lose its valence electrons to achieve a stable electron configuration. The. How Many Electrons Does Zinc Lose.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? How Many Electrons Does Zinc Lose in the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Zinc atom electron configuration through orbit. Zinc is the metal transition element and hence it belongs to the d. valency of zinc. By losing its two 4s electrons, zinc. another implication is that zinc. How Many Electrons Does Zinc Lose.
From www.pinterest.com
22 best Lessons to learn images on Pinterest School projects, Atomic How Many Electrons Does Zinc Lose table of contents. What is the electron configuration of zinc? This means that neutral zinc atom has a total of. therefore, the number of electrons in neutral atom of zinc is 30. The precise and accurate valency of zinc is +2. valency of zinc. Each electron is influenced by the electric fields produced by the positive nuclear. How Many Electrons Does Zinc Lose.